Research
Research field: Biomaterials, Medical Devices, Drug Delivery Systems (DDS), and Regenerative Medicine
The field of biomaterials encompasses a diverse array of materials utilized in clinical applications to enable effective treatment, diagnosis, and prevention of diseases. As a dynamic and continually advancing discipline, biomaterials research integrates engineering, medicine, and pharmaceutical sciences, making it inherently interdisciplinary. A comprehensive understanding of the associated phenomena necessitates an integrative approach, incorporating perspectives not only from chemistry but also from physics and biology.
Our research is motivated by pressing clinical needs and is centered on the molecular-level design and development of polymer-based biomaterials. These biomaterials are intended for applications in medical devices, drug delivery systems (DDS), and regenerative medicine, including tissue engineering. We place particular emphasis on advancing the following three academic methodologies:
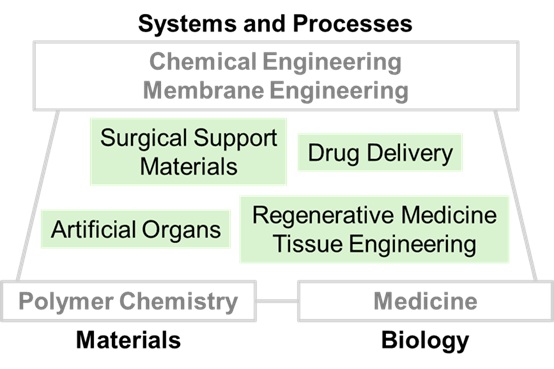
Academic Methodologies: Chemical Engineering, Basic Medical Science, and Polymer Chemistry
Chemical Engineering
Our laboratory specializes in chemical and membrane engineering, with a particular emphasis on basic medical sciences and polymer chemistry. The design and optimization of therapeutic devices require a systematic approach based on fundamental principles of chemical engineering, which encompass mass, heat, and momentum transfer, as well as chemical reaction dynamics.
Moreover, it is essential to adopt an integrated perspective that treats synthetic materials and biological tissues as a cohesive system. Key phenomena frequently occur at the interfaces between artificial materials and biological tissues, making membrane engineering vital for addressing mass transfer and chemical interactions at these critical junctions.
Basic Medical Science
The development of advanced biomedical materials necessitates a thorough understanding derived from basic medical sciences and biology. When biomaterials—or biomaterials loaded with drugs or cells—are administered into the body for therapeutic purposes, complex responses are elicited at cellular and systemic levels.
These responses, including inflammation, coagulation, fibrinolysis, and fibrosis, must be meticulously studied from a life sciences perspective. As biomaterials are also utilized as carriers for drugs or cells, and as scaffolds, it becomes crucial to investigate how cells interact with their interior and surface. Ultimately, therapeutic efficacy must be quantitatively assessed using principles grounded in medical science.
Polymer Chemistry
Biological systems are governed by the orchestrated activities of highly structured and functional biomolecules such as polysaccharides, proteins, nucleic acids, and lipids—among which polysaccharides, proteins, and nucleic acids are classified as biopolymers.
In our laboratory, we explore the design and development of novel biomaterials using these biomolecules as raw materials, focusing on polymer synthesis and the analysis of polymer physical properties. Our design philosophy is strongly inspired by the intricate hierarchical structures, functions, and rational designs inherent in biological systems, utilizing concepts from biomimetic chemistry and bio-inspired chemistry to achieve innovative solutions.
Applied Research: Medical Devices, Drug Delivery Systems (DDS), and Regenerative Medicine
We are committed to applied research aimed at advancing disease treatment, primarily through collaborative efforts with the University of Tokyo Hospital and other leading medical institutions.
Drug Delivery Systems (DDS)
Drug delivery systems (DDS) are advanced technologies designed to deliver "the minimum necessary amount of a drug to the targeted tissues at the optimal timing." A key principle of DDS is the controlled release of drugs. When drug concentrations exceed the therapeutic range, adverse side effects may occur; conversely, subtherapeutic levels may render the drug ineffective. Maintaining drug concentrations within the therapeutic window for prolonged durations enhances therapeutic efficacy, minimizes side effects, reduces dosing frequency, and improves patient quality of life.
Our research is primarily focused on localized drug delivery strategies, particularly for diseases such as peritoneal dissemination of gastric and ovarian cancers, biliary tract cancer, pancreatic cancer, mesothelioma, scleroderma, and keloids. Additionally, recent advancements in biosensor technology, wireless power transfer, and digital health innovations have made on-demand DDS—where drug release is triggered by external stimuli such as magnetic fields or ultrasound—increasingly viable. We are actively pursuing research in this emerging domain to push the boundaries of drug delivery technology.

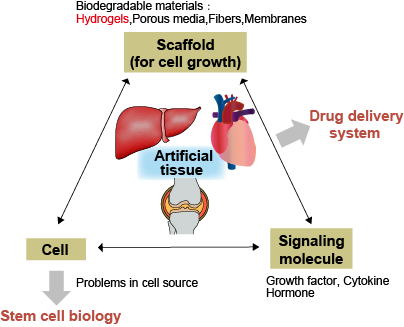
Regenerative Medicine and Tissue Engineering
Tissue engineering seeks to regenerate functional tissues through the integration of three essential components: cells, scaffold materials, and soluble factors, while carefully controlling the engineering processes. Injectable hydrogels, microcapsules, and gel microwells are promising candidates for use as transplantable scaffolds.
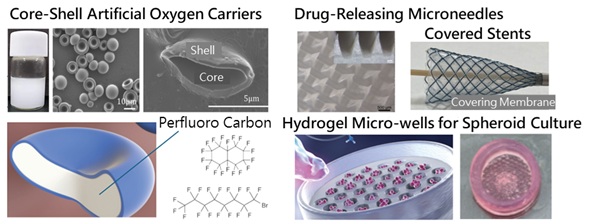
Our current research focuses on pancreatic islet and bone regeneration using hydrogels, emphasizing key factors such as cell differentiation, functional expression following transplantation, and angiogenesis. A critical challenge in three-dimensional tissue construction is ensuring adequate oxygen supply. To address this, we are developing an innovative core-shell artificial oxygen carrier that mimics the morphology and flexibility of human red blood cells. This novel carrier comprises an elastomer shell encasing a perfluorocarbon core, a material known for its high oxygen solubility. Such developments support advancements in 3D tissue regeneration and facilitate the preservation of tissues and organs.
Medical Devices (Anti-adhesion Materials, Anti-stenosis Agents, Tissue Adhesives, Wound Dressings)
After open or laparoscopic surgery, organs can adhere to each other or to the peritoneum, leading to a condition known as peritoneal adhesions. This issue is particularly common following liver cancer resection, often resulting in reduced surgical safety and prolonged operative times. To mitigate these risks, we are actively developing innovative anti-adhesion materials.
In pancreatic cancer surgery, postoperative complications such as pancreatic fistula—caused by pancreatic juice leakage at the surgical site—represent significant clinical challenges. Accordingly, we are designing effective materials to prevent pancreatic fistulas. Furthermore, gastrointestinal strictures that frequently arise after procedures like endoscopic submucosal dissection (ESD) highlight the importance of developing reliable anti-stenosis materials.
Hemostasis and tissue repair are indispensable in various surgical procedures. To address these needs, we are advancing research into effective hemostatic agents and wound dressings. Such medical devices are critical to ensuring safe and efficient surgical outcomes.
In addition, we are exploring combination products that merge DDS technologies with innovative medical device designs, as well as cutting-edge approaches that integrate regenerative medicine techniques, such as cell sheet applications. Our research remains resolutely focused on addressing real-world clinical challenges through the development of transformative technologies and solutions.
- Research Topic 1: Development of Biomedical Hydrogels
- Research Topic 2: Development of Biomedical Membranes and Biomedical Microparticles
Research Topic 1: Development of Biomedical Hydrogels
Injectable hydrogels, which can be delivered via syringes or catheters, hold immense promise for applications in minimally invasive therapies. By employing naturally derived polysaccharides and proteins as structural frameworks and utilizing biocompatible chemical reactions, a diverse array of hydrogels can be synthesized. Their ease of administration through laparoscopes, flexible endoscopes, and surgical robots renders these hydrogels highly versatile, with potential applications spanning regenerative medicine, drug delivery carriers, anti-adhesion materials, anti-stenosis materials, hemostatic agents, and tissue adhesives.
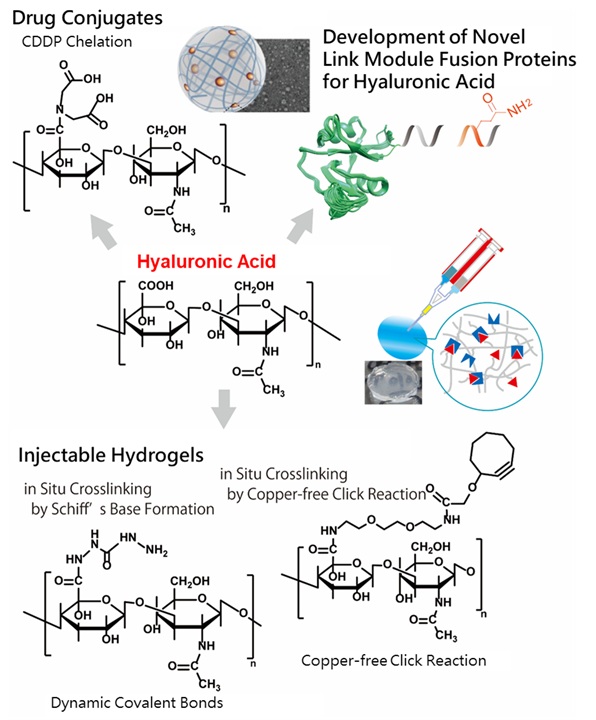
Hyaluronic acid, a naturally occurring polysaccharide and a major component of the extracellular matrix (ECM), exhibits unique viscoelastic properties, excellent water retention capacity, and exceptional biocompatibility. Our research focuses on the design and development of hydrogels utilizing click chemistry and dynamic covalent bonding, emphasizing shear-thinning and self-healing properties.
The potential of hydrogels and polysaccharides in drug delivery systems (DDS) is also a key area of investigation. Recent advancements in antibody-drug conjugates (ADCs)—where antibodies are linked to drugs via specialized linkers—have catalyzed rapid progress in this field. We are actively working on polysaccharide-based drug conjugates, which have the intrinsic ability to form physical crosslinks, enabling the creation of nanoscale gels.
Furthermore, hyaluronic acid interacts with specific proteins such as CD44, TSG-6, and Aggrecan, all of which contain a conserved structural motif known as the "link module." By incorporating protein engineering techniques, we conduct detailed investigations into hyaluronic acid-binding proteins.
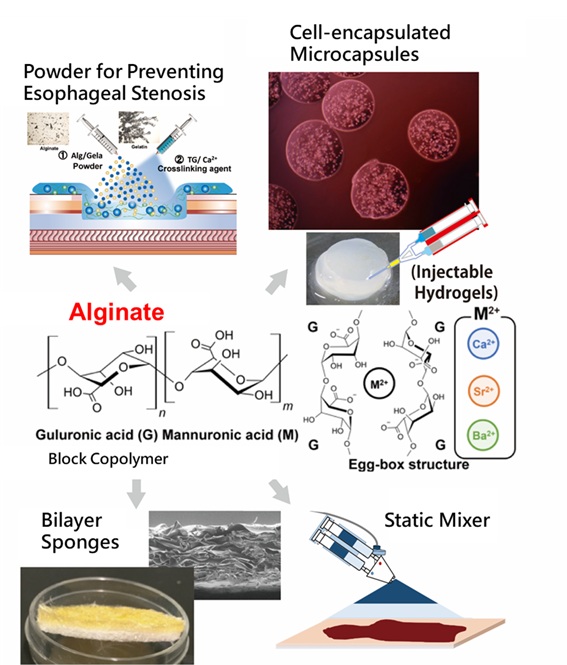
Alginate, a polysaccharide derived from natural sources such as kelp, forms a distinctive chelate structure known as the "egg-box" structure in the presence of divalent calcium ions (Ca²⁺). This property allows alginate to rapidly form gels with excellent moldability, high biocompatibility, and safety, making it an ideal material for cell encapsulation.
The functionality and performance of medical devices are heavily influenced by the methods of hydrogel delivery. For instance, administering hydrogels in the form of sprays or powders can substantially enhance therapeutic outcomes and operational convenience. Beyond material development, adopting a chemical engineering perspective is essential. Our work in this area involves the use of tools such as static mixers, electrosprays, and atomizers, as well as the study of toroidal gel particles. With the rapid progress in additive manufacturing (AM) technologies, particularly 3D printing, we are also advancing the development of hydrogel-based bioinks.
Additionally, our research explores the utilization of a wide range of natural polysaccharides, including chitosan, dextran, carboxymethyl cellulose, xanthan gum, guar gum, and heparin, as well as proteins such as collagen, gelatin, and albumin.
Research Topic 2: Development of Biomedical Membranes and Biomedical Microparticles
In drug delivery systems (DDS), controlled drug release is fundamentally a mass transfer process within materials, with the design of formulations such as transdermal patches and wound dressing grounded in membrane engineering principles. Artificial organs, including artificial lungs and kidneys, are modular systems composed of hollow fiber membranes, and their development is similarly rooted in membrane engineering. Additionally, in biopharmaceutical manufacturing processes, various separation membranes are widely utilized, such as reverse osmosis membranes for pure water production, ultrafiltration membranes, and microfiltration membranes for purification.
Drying, categorized as a unit operation in separation engineering, plays a critical role in the production of biomedical materials. Our laboratory conducts research on biodegradable porous sponges fabricated using freeze-drying techniques, alongside collaborative studies on microneedles. We also focus on the development of covering membranes for covered stents, which are employed in vascular and gastrointestinal treatments. Covering membranes typically rely on highly elastic materials to meet functional requirements.
Various methods exist for the fabrication of microparticles, and our primary approach is the SPG membrane emulsification technique. SPG membranes are glass porous membranes characterized by uniformly sized pores adjustable within the range of 0.5 to 30 µm. This method produces highly uniform emulsions by driving a dispersed phase through the membrane pores into a continuous phase. Leveraging this technique, we are developing microparticles composed of poly(lactic-co-glycolic acid) (PLGA) capable of releasing enzymes or anticancer agents, as well as core-shell or hydrogel-type artificial oxygen carriers (artificial red blood cells), considering that the diameter of human red blood cells is approximately 8 µm.
Liposomes are spherical vesicles formed by phospholipids exhibiting amphiphilic properties, which give rise to a bilayer structure analogous to cell membranes. They have long been investigated as drug delivery carriers, and our research also encompasses studies on liposomes.
By advancing and broadening membrane engineering for medical applications, our goal is to develop innovative technologies that contribute significant value to clinical practice.